Résumés Année 2002
"Molecular tectonics: abiotic control of hydroxyapatite crystals morphology"
V. BALL, J.-M. PLANEIX, O. FELIX, J. HEMMERLE, P. SCHAAF, M. W. HOSSEINI, J.- C. VOEGEL, Cryst. Growth and Design, 2002, 2, 489-492.(download .pdf)
Abstract: Ultra flat hydroxyapatite platelets with characteristic lateral dimensions in the µm range and a thickness of a few nanometers were synthesized at ambient temperature from supersaturated calcium and phosphate solutions and in the presence of a bis-amidinium cation.
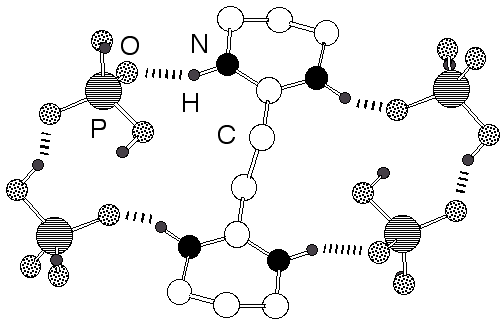 | 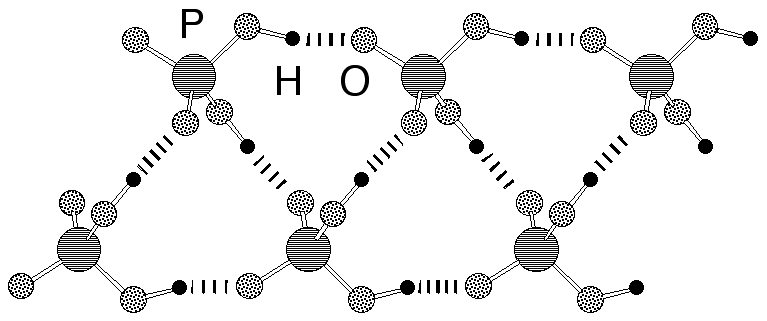 | 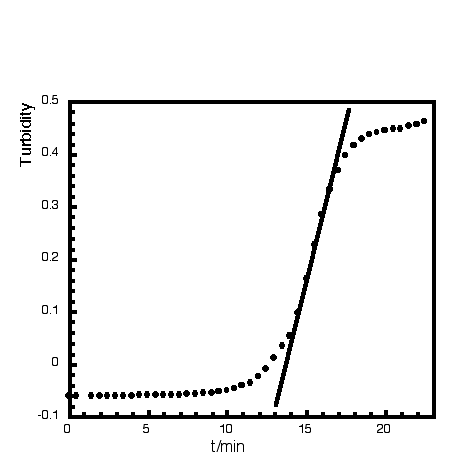 |
"Photophysical, electrochemical and electrochromic properties of copper bis-((4,4'-dimethyl-6,6'-diphenyl-2,2'-bipyridyl) complexes"
R. M. WILLIAMS, L. DE COLA, F. HARTL, J.-J. LAGREF, J.-M. PLANEIX, A. DE CIAN, M. W. HOSSEINI, Coord. Chem. Rev., 2002, 230/1-2, 243-251.(download .pdf)
Abstract: The synthesis, solution and solid state structural characterization, photophysical and electrochemical properties of two redox forms of an electrochromic copper-bis(4,4'-dimethyl-6,6'-diphenyl-2,2'-bipyridine) complex, [Cu(3)2]n (n = +1, +2), are presented. Both complexes were characterized in the solid state by X-ray diffraction methods on single-crystals showing that both forms exist in a pseudo-tetrahedral coordination, and a comparison with other structures was made. Like most copper(I) complexes, the red [Cu(3)2]+ complex shows a rather weak emission (Φem= 2.7 x 10-4, dichloromethane). The lifetime of the emitting MLCT state is 34 ± 1 ns, as observed with time resolved emission, and transient absorption (in deoxygenated dichloromethane). Typical emission and transient absorption spectra are presented. The transient absorption spectra indicate that the MLCT state absorbs stronger than the ground state, which is relatively uncommon for metal bipyridine complexes, i.e. no ground state bleaching is observed. The green [(3)2Cu]2+ complex does not show any observable emission or transient absorption, which is a common feature for Cu(II) complexes of this type. The electronic absorption spectra of the chemically and electrochemically produced copper(I/II) complexes are identical. The repeated electrochemical conversion of the Cu(I) center into Cu(II) and vice versa does not cause any decomposition. This is consistent with a fully reversible CuI/CuII redox couple in the corresponding cyclic voltammogram, (E1/2 (CuI/CuII)= + 0.68 V vs SCE = + 0.23 V vs Fc/Fc+). These observations indicates that no large structural reorganization occurs upon electrochemical timescales (sub second), and that the different ways of generating the complexes does not effect their final structure, apart from the small differences observed in the X-ray structures of both forms. These characteristics make these complexes rather well suited for their incorporation into an electrochromic display configuration.
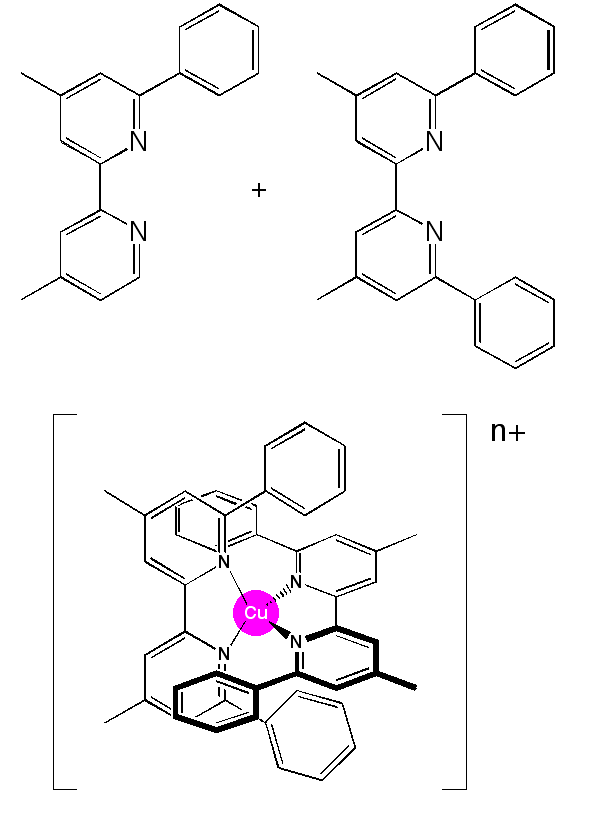 |  |  |
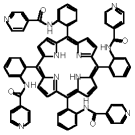
"1- and 2-D coordination networks based on porphyrin and cooper: an example of supramolecular isomerism"
B. ZIMMER, V. BULACH, M. W. HOSSEINI, A. DE CIAN, N. KYRITSAKAS, Eur. J. Inorg. Chem., 2002, 3079-3082. (download .pdf)
Abstract: A high yield synthesis of atropoisomers of the meso-tetrakis(o-isonicotinoylamidophenyl)porphyrin was achieved. Depending on the crystallising solvent system, the a2b2 atropoisomer leads to the formation of either 1-D or 2-D coordination networks in the presence of Cu(OAc)2.
"Second Sphere Supramolecular Chirality: Racemic Hybrid H-Bonded 2-D Molecular Networks"
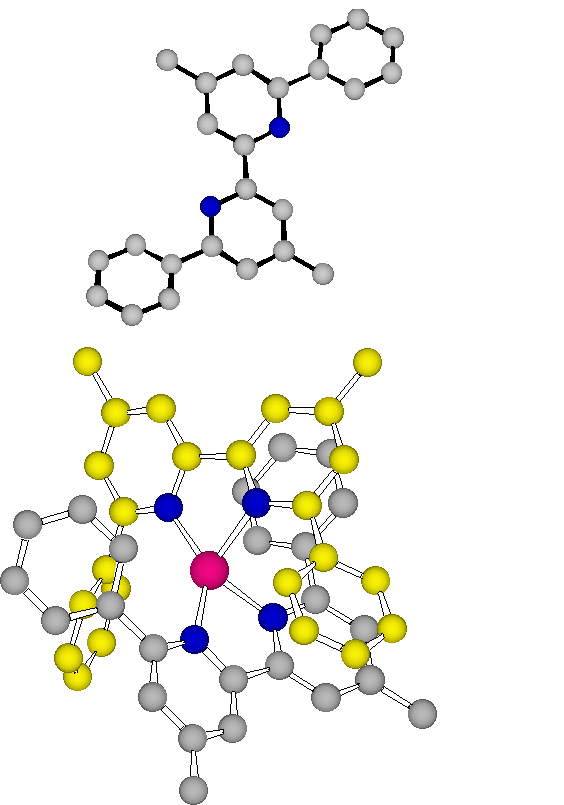
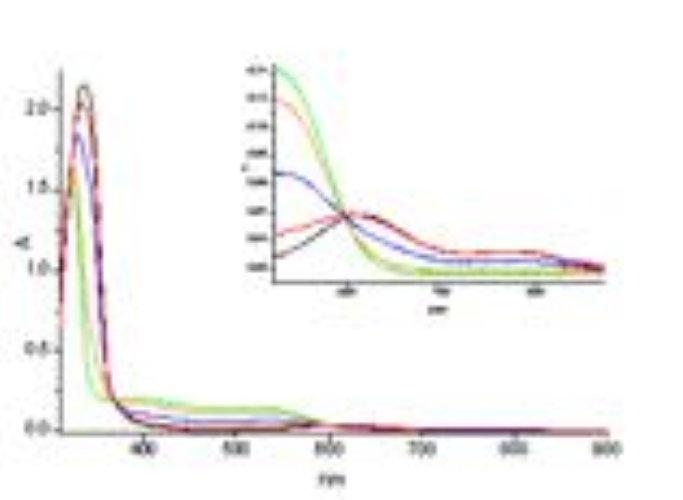
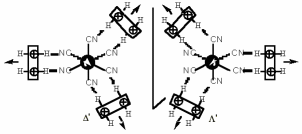
S. FERLAY, O. FELIX, M. W. HOSSEINI, J.-M. PLANEIX, N. KYRITSAKAS, J. Chem. Soc., Chem. Commun., 2002, 702-703. (download .pdf)
Abstract: Neutral hybrid 2D networks have been generated using a bis-amidinium capable of chelating M(CN)63- anions via hydrogen bonds. The packing of the achiral 2-D networks leads to channels which are occupied by water molecules forming polymeric H-bonded chains. Furthermore, due to the dihapto mode of H-bonding, the presence of supramolecular ch irality of the D and ? type taking place within the second coordination sphere of the metallic centre was demonstrated.
 |  |
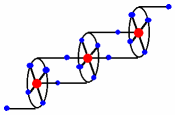
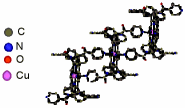
"Coordination polymers based on porphyrin and copper: the influence of the crystallization solvents on the dimensionality of the network"
B. ZIMMER, M. HUTIN, V. BULACH, M. W. HOSSEINI, A. DE CIAN, N. KYRITSAKAS, New. J. Chem., 2002, 26, 1532-1535. (download .pdf)
Abstract: The a2b2 atropoisomer of meso-tetrakis(o-nicotinoylamidophenyl)-Copper porphyrin leads in the presence of Cu(II) cation to coordination polymers in the crystalline phase. The networks are generated by interconnection of metallporphyrin units through the coordination of Cu centres adopting an octahedral coordination geometry by two pyridines belonging to consecutive complexes. Depending on the solvent systems used, the 1-D coordination network based on 1a2b and Cu dication was shown to form a 3-D network based on a combination of three distinct supramolecular forces.
 |  |  |
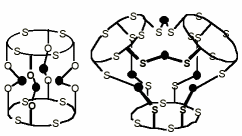
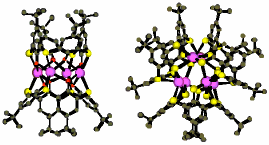
"Koilands from thiophiles: mercury (II) clusters from thiacalixarenes"
H. AKDAS, E. GRAF, M. W. HOSSEINI, A. DE CIAN, A. BILYK, B. W. SKELTON, G. A. KOUTSANTONIS, I. MURRAY, J. M. HARROWFIELD, A. H. WHITE, J. Chem. Soc., Chem. Commun., 2002, 1042-1043. (download .pdf)
Abstract: Tetra- and hexa-nuclear mercury(II) complexes have been obtained from tetrathiacalix[4]arene and tetramercaptotetrathiacalix[4]arene, respectively, and structurally characterised in the solid state. The complexes provide new digonal and trigonal receptors of the koiland type.
 |  |
"Non-centrosymmetric packing of 1-D coordination networks based on chirality"
A. JOUAITI, M. W. HOSSEINI, N. KYRITSAKAS, J. Chem. Soc., Chem. Commun., 2002, 1898-1899. ( download .pdf )
Abstract: Using CoCl2 units and a chiral tecton possessing C2 chirality and based on two coordination poles composed of a pyridine unit connected at the 4-position to a pyridine bearing at the 2 and 6 positions t wo optically active oxazoline moieties, a polar solid is obtained. The latter results from the acentric packing of directional 1-D coordination networks.
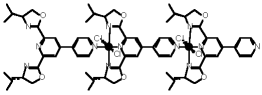 |  |
"Design, synthesis and solid state structural characterisation of a metallacyclophanes formed by a diazadioxamacrocycle bearing two pyridines and silver (I) cation"
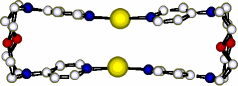
V. JULLIEN, M. W. HOSSEINI, J.-M. PLANEIX, A. DE CIAN, J. Org. Met. Chem., 2002, 643-644, 376-380. (download .pdf)
Abstract: functionalisation of 1,7-diaza-4,10-dioxacyclododecane 2 on both nitrogen atoms by two pyridine units using the para-position with respect to the nitrogen of the pyridine, the bismonodentate ligand 1 was obtained and structurally characterised in the solid stat e by X-ray diffraction on single-crystal. Under self-assembly condition, in the presence of AgPF6, the above mentioned ligand 1 forms the macrotricyclic metallamacrocycle 4 composed of two ligands and two silver cations with linear coordination of both metal cations. The barrel type structure thus obtained was also characterised in the solid state by X-ray diffraction methods.
 |  |
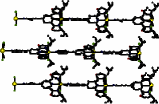
"Molecular beskets based on tetramercaptotetrathiacalix[4]arene and tetrathiacalix[4]arene"
H. AKDAS, L. BRINGEL, V. BULACH, E. GR AF, M. W. HOSSEINI, A. DE CIAN, Terahedron Lett., 2002, 43, 8975-8979. (download .pdf)
Abstract: Upon treatment of p-tbut-tetrathiacalix[4]arene and p-tbut-tetrathiatetramercaptocalix[4]arene by 1,2-dibromoethane in the presence of K2CO3 two new basket-type derivatives are obtained and their structures characterised in the solid state by X-ray diffraction on single crystals.
"Design of 3-D Coordination Networks: Topology and Metrics"
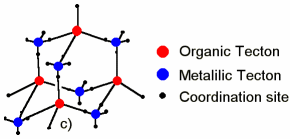
S. FERLAY, S. KOENIG, M. W. HOSSEINI, J. PANSANEL, A. DE CIAN N. KYRITSAKAS, J. Chem. Soc., Chem. Commun., 2002, 218-219. (download .pdf)
Abstract: Using 4, 4', 4''- tricyanotriphenylmethanol 1 as a hetero tetradentate tecton with c3v symmetry bearing three CN and one OH groups, under self-assembly conditions a 3-D coordination network was obtained in the presence of Ag+ cation acting as a tetrahedral metallic tecton. Due to the metrics of 1 (three lo ng and one short di stances between the central C atom and N and O coordination sites respectively), the 3-D network is of pseudo-diamondoid type with different cavity sizes. Although a two-fold homo-interpenetration is observed for the 3-D networks, the remaining space is occupied by one CHCl3 and four MeOH solvent molecules.
 |  |
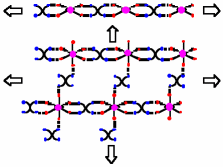
"Molecular tectonics and supramolecular chirality: rational design of hybrid 1-D and 2-D H-bonded molecular networks based on bisamidinium dication and metal cyanide anions"
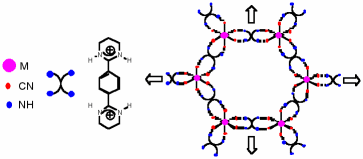
S. FERLAY, V. BULACH, O. FELIX, J.M.PLANEIX, M. W. HOSSEINI, N. KYRITSAKAS, Cryst. Eng. Commun., 2002, 4, 447-453. (download .pdf)
Abstract: Using the bis-amidinium dication 1 bearing four acidic protons disposed in a divergent fashion and capable of chelating through H-bonds metal cyanide anions neutral 1- and 2-D hybrid networks were generated. Whereas in the presence of M(CN)42- (M = Ni(II), Pd(II) and Pt(II)), 1-D H-bonded networks are formed through a double dihapto mode of H-bonding between the dication and the dianion, in the case of M(CN)64- (M = Fe(II) and Ru(II)), 2-D networks based on the interconnection by the dication 1 of 1-D networks analogous to those formed in the case of []M(CN)4]2- mentioned above are obtained. Interestingly, in the case of [M(CN)6]3- (M = Cr(III)), again a 2-D H-bonded network is formed through the formation of three dihapto mode of H-bonding between the dication and the trianion. Due to this dihapto or chelate mode of H-bonding, a supramolecular chirality of the and ' type is generated within the second coordination sphere of the metallic centres. The packing of the achiral 2-D networks thus obtained leads to channels which are occupied by water molecules forming polymeric H-bonded chains.
 |  |
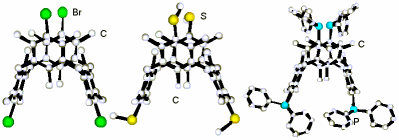
"Synthesis and Solid State Structural Analysis of Exotetradentate Ligands Based on Mesit ylene-Derived [1.1.1.1 ] metacyclophane Blocked in 1,3-Alternate."
C. KLEIN, E. GRAF, M. W. HOSSEINI, A. DE CIAN, N. KYRITSAKAS-GRUBER, Eur. J. Org. Chem., 2002, 802-809. (download .pdf)
Abstract: A series of new tetradentate ligands based on the [1.1.1.1] metacyclophane backbone blocked in the 1,3-alternate was achieved. For the strategy developed, the common synthon for the preparation of twelve ligands bearing fou r interaction sites occupying the apices of a pseudo tetrahedron (H, SH, CN, SCH3, CO2H, CHO, PPh2, P(O)Ph2, NO2, NH2, pyridine and para-methoxyphenyl) was the tetrabromo-derivative 7. In addition to classical characterisation methods applied to all reported ligands, compounds 7, 9, 12 and 13 were structurally studied in the solid state by X-ray diffraction on single-crystal which indeed confirmed their 1,3-alternate conformation.
 |  |
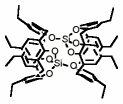
"Molecular tectonics : Design and structural analysis of 1-D and 2-D self-inclusion molecular networks in the crystalline state"
J. MARTZ, E. GRAF, M. W. HOSSEINI, A. DE CIAN, N. KYRITSAKAS-GRUBER, C. R. Chimie, 2002, 5, 481-486. (download .pdf)
Abstract: Th e synthesis of the new symmetrical koiland 2 based on the double fusion of two p-ethylcalix[4]arene in cone conformation by two silicon atoms was achieved and its structure confirmed by X-ray diffraction on a single crystal. Koiland 2, a concave building block possessing two calix type preorganised cavities disposed in a divergent fashion as well as ethyl groups as potential connector moieties, affords either a discrete exo-binuclear inclusion complex in the solid state in the presence of p-xyl ene acting as a stopper or infinite 1-D or 2-D self-inclusion networks or koilates mainly based on van der Waals interactions and resulting from the inclusion processes taking place between concave and convex moieties of consecutive koilands.
 |  |  |


