Résumés Année 1998
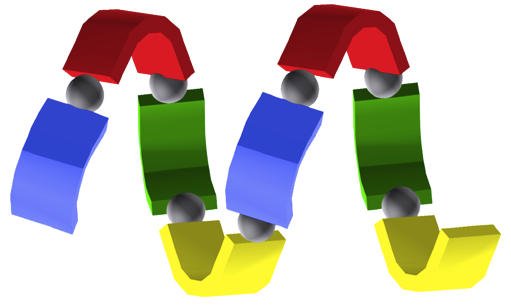
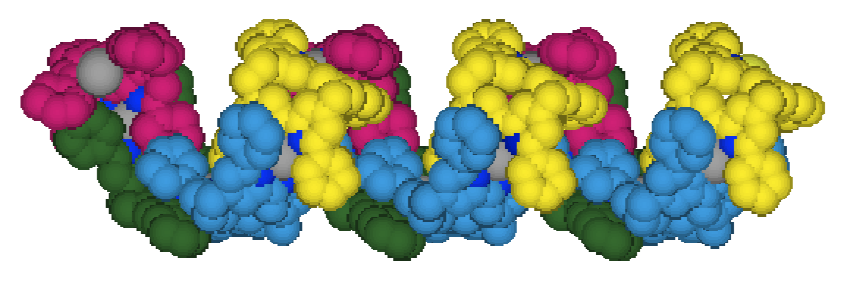
"Synthesis and Structural Analysis of a Helical Coordination Polymer Formed by the Self-assembly of a 2,2'-bipyridine based Exoditopic Macrocyclic Ligand and Silver Cations"
C. KAES, M. W. HOSSEINI, C. E. F. RICKARD, B. W. SKELTON, A. H. WHITE Angew. Chem. Int. Natl. Ed. Engl., 1998, 37, 920-922.(download .pdf)
Abstract : The overall topology of coordination polymers can be controlled by means of the coordination preferences of the metal center and the structure of the bridging ligand. This is demonstrated here by the synthesis of a single-stranded helical coordination polymer by the self-assembly of the exo-ditopic ligand 1 and silver ions.
 |  |  |
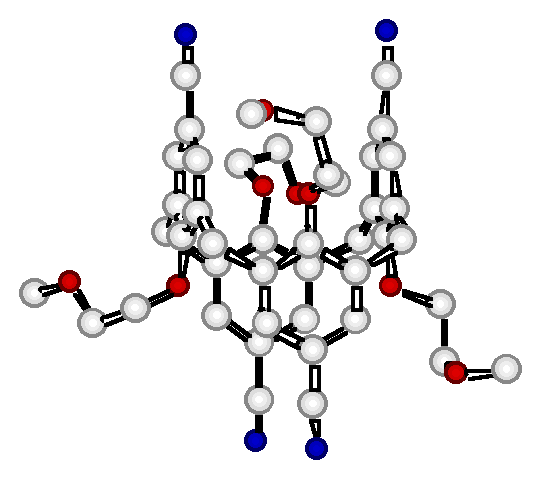
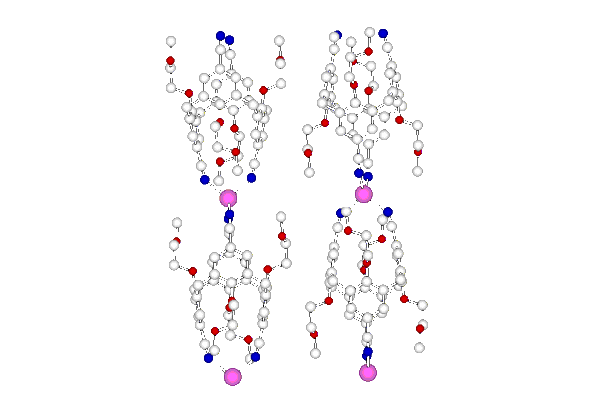
"Synthesis and structural analysis of an infinite linear coordination network formed by the self-assembly of tetracyanocalix[4]arene ligands and silver cations"
G. MISLIN, E. GRAF, M. W. HOSSEINI, A. DE CIAN, N. KYRITSAKAS, J. FISCHER, Chem Comm., 1998, 2545-2546. (download .pdf)
Abstract : Using the O-alkylation of the lower rim of the calix[4]arene to impose the 1,3-alternate conformation and the functionalisation of the upper rim to set-up four nitrile groups pointing in a divergent fashion, an exo-ligand capable of forming linear coordination networks was obtained. Upon self-assembly of the latter and silver cation, a linear coordination polymer was obtained and structurally analysed in the solid state by an X-ray study.
 |  |  |
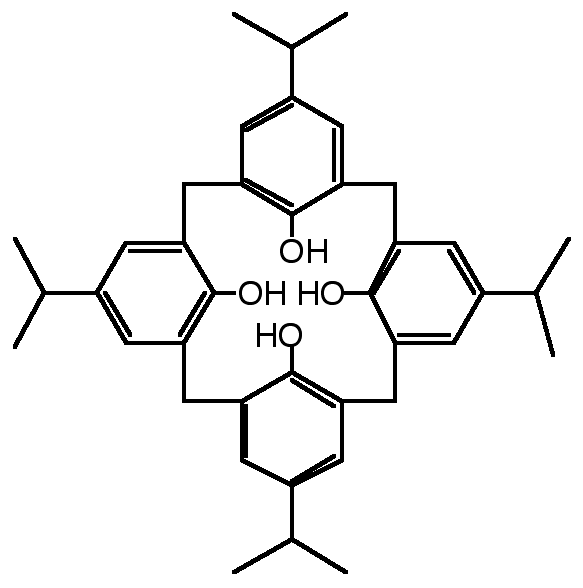
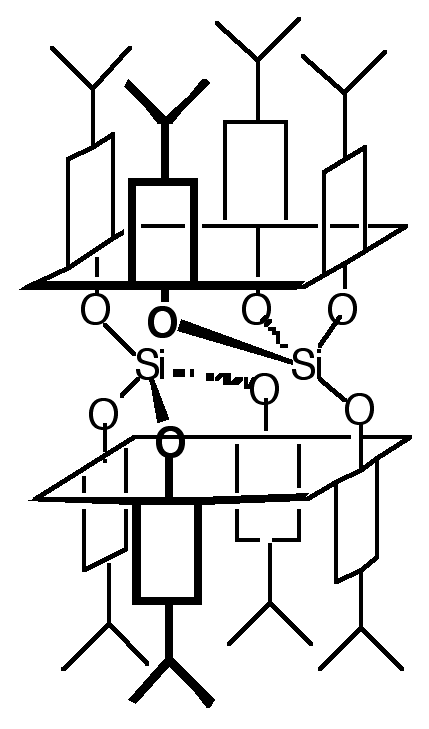
"Molecular tectonics: design, synthesis and structural analysis of a molecular network based on inclusion processes using a doubly fused p-isopropylcali x[4]arene"
J. MARTZ, E. GRAF, M. W. HOSSEINI, A. DE CIAN, N. KYRITSAKAS, J. FISCHER, J. Mat. Chem., 1998, 8, 2331-2333. (download .pdf)
Abstract : The synthesis of a hollow molecular module (koiland) possessing two divergent cavities was achieved by double fusion of two p-isopropylcalix[4]arenes in cone conformation by two silicon atoms. The formation of either a discrete binuclear inclusion complex in the presence of CH2Cl2 acting as stopper or of an infinite 1-D inclusion network (koilate) in the presence of hexadiyne acting as connector was demonstrated in the solid state by single-crystal diffraction studies. The inclusion network was based on the interconnection of consecutive koilands by connector molecules.
 |  |  |
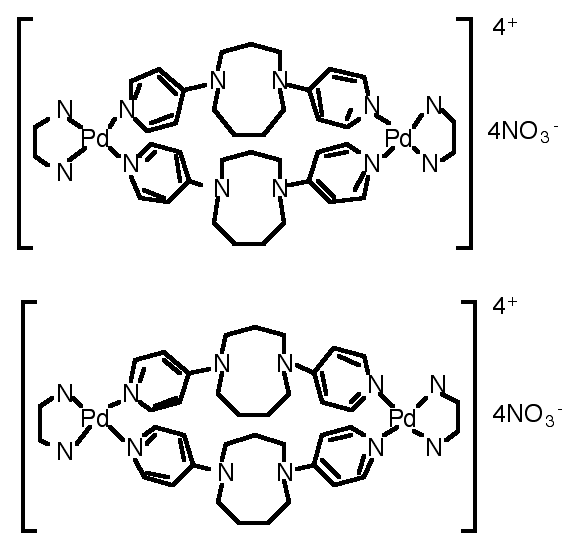
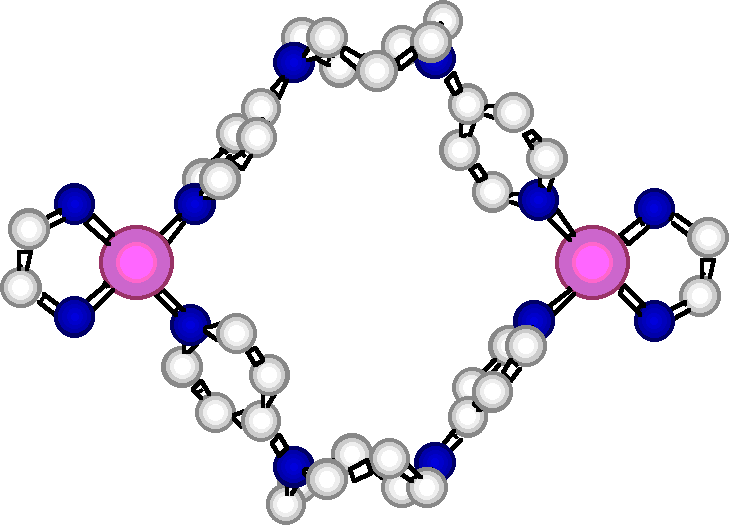
"Exo-ligands based on two p-aminopyridine interconnection by tuneable alkyl chains: design, synthesis and structural analysis of silver and palladium metallamacrocycles"
R. SCHNEIDER, M. W. HOSSEINI, J.-M. PLANEIX, A. DE CIAN, J. FISCHER, Chem. Comm., 1998, 1625-1626. (download .pdf)
Abstract : Exo-ligands based on functionalisation of diazacyclic cores of variable size by two pyridines was achieved. Under self-assembly conditions, their ability to form metallamacrocycles in the presence of palladium or silver metals was demonstrated by X-ray crystallography.
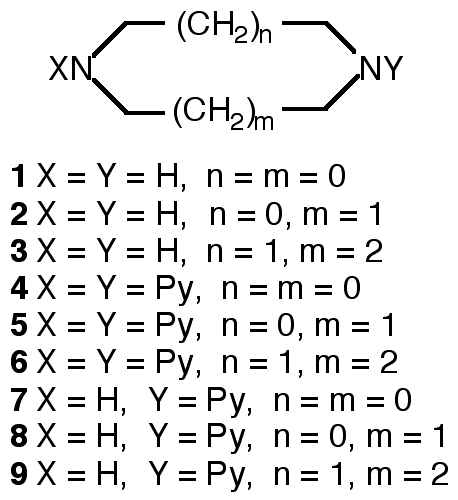 |  |  |
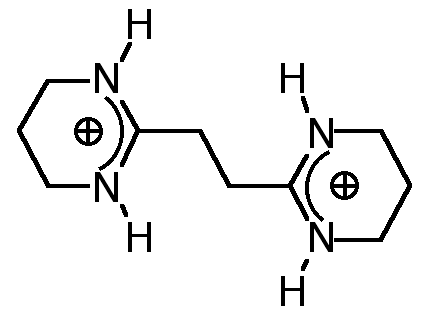
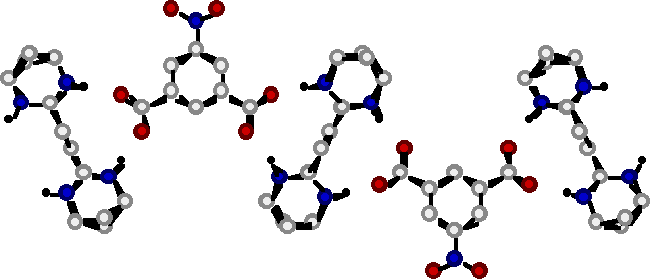
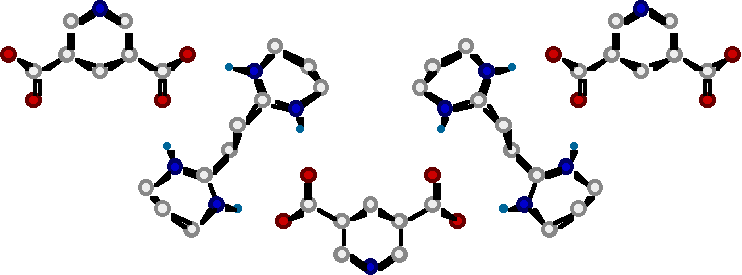
"Molecular Tectonics VIII : Formation of 1D- and 3-D Networks based on the simultaneous use of hydrogen bonding and ionic interactions"
O. FELIX, M. W. HOSSEINI, A. DE CIAN, J. FISCHER, N. J. Chem., 1998, 22, 1389-1393. (download .pdf)
Abstract : Using a combination of directional hydrogen bonding and ionic interactions 1- and 3-D molecular networks were obtained in the crystalline phase. It has been shown that a bis amidinium dication and dicarboxylate mono or dianions form one and two dimensional networks in the solid state. The formation of networks was based on a dihapto mode of hydrogen bonding between the complementary dication and dianion.
 |  |  |
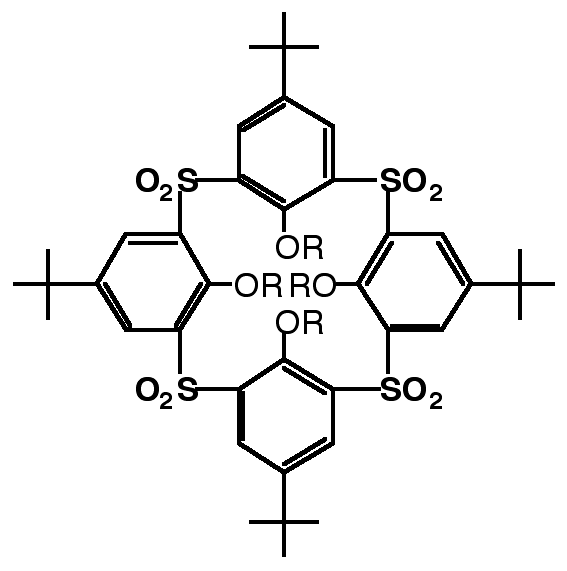
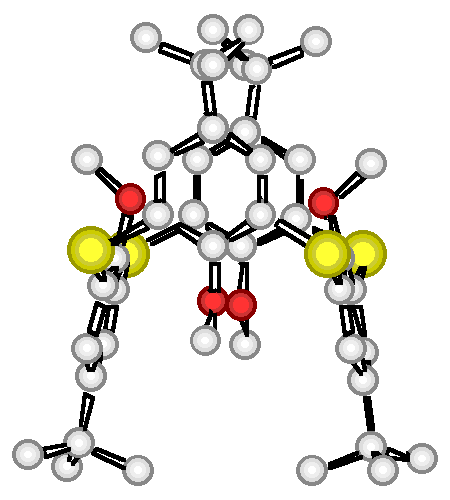
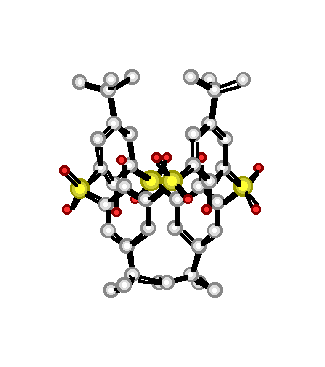
"Sulfone-calixarenes : a new class of molecular building block"
G. MISLIN, E. GRAF, M. W. HOSSEINI, A. DE CIAN, J. FISCHER, Chem. Comm., 1998, 1345-1346 (download .pdf)
Abstract : The synthesis of a new calix[4]arene derivative based on the linkage of the phenolic rings by four sulfone groups was achieved and its 1,3-alternate conformation in the crystalline phase, resulting from both inter- and intra-molecular H-bonding, was established by crystallography.
 |  |  |
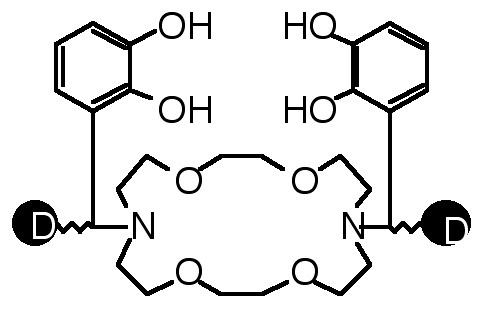
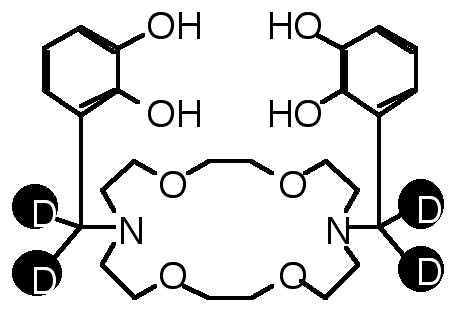
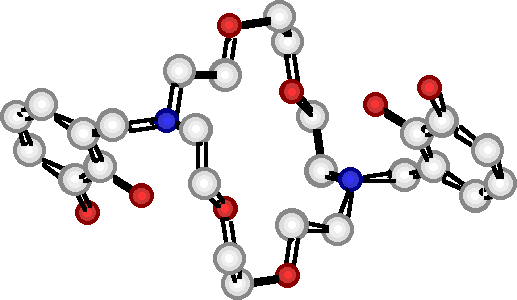
"Deuterium Labelled Borocryptands : Synthesis, Structural Analysis and Binding Studies"
E. GRAF, M. W. HOSSEINI, A. DE CIAN, J. FISCHER, Tetrahedron Lett., 1998, 39, 3501-3504. (download .pdf)
Abstract : High yield syntheses of two new deuterium labelled compound 2 and 3 based on the [22] macrocyclic backbone beari n g two catechol units was achieved. The solid state structure of the tetradeuterated compound 3 was elucidated by X-ray diffraction methods. The binding ability of 2 and 3 towards both boron and alkaline metal cations was established in solution by 1H-, 2D- and 11B-NMR spectroscopy.
 |  |  |
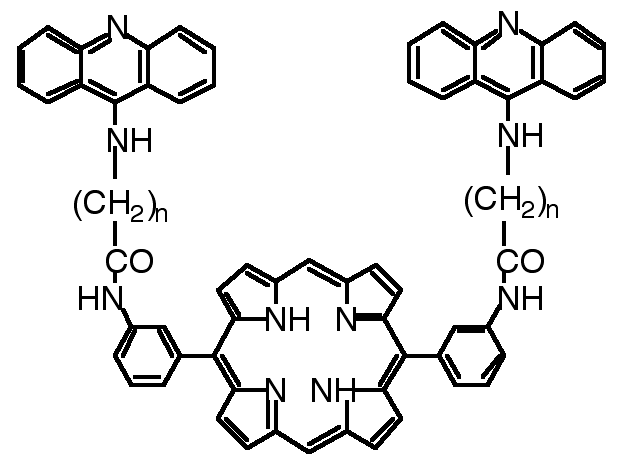
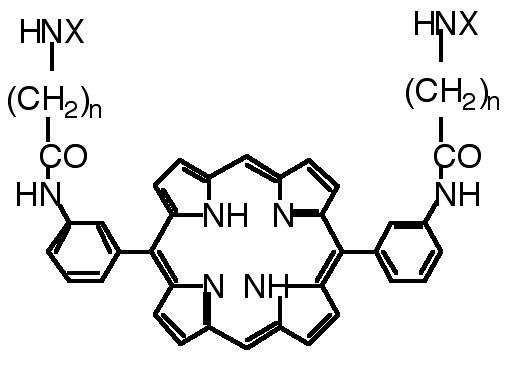
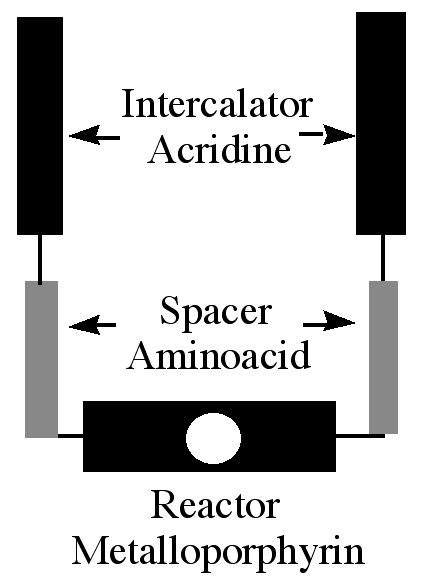
"Design, synthesis and cleaving activity of an abiotic nuclease based on a MnIII-porphyrin complex bearing two acridine moieties"
C. DREXLER, M. W. HOSSEINI, G. PRATVIEL, B. MEUNIER, Chem. Comm., 1998, 1343-1344. (download .pdf)
Abstract : The synthesis of meso-5,15-bis(meta-amino-phenyl)porphyrin bearing two acridine derivatives and of its Mn(III) complex was achieved. In the presence of oxygen atom donors, the metalloporphyrin was shown to cleave double-stranded DNA.
 |  |  |
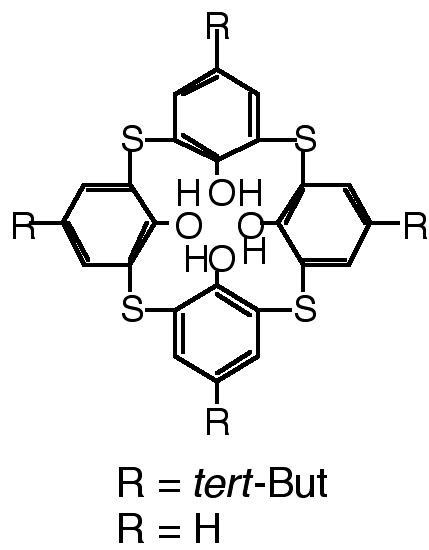
"Thiacalixarenes : Synthesis and Structural Analysis of Thiacalix[4]arene and of p-tert-Butylthiacalix[4]arene"
H. AKDAS, L. BRINGEL, E. GRAF, M. W. HOSSEINI, G. MISLIN, J. PANSANEL, A. DE CIAN, J. FISCHER, Tetrahedron Lett., 1998, 39, 2311-2314. (download .pdf)
Abstract : The synthesis of tetrathiacalix[4]arene was achieved by the detertiobutylation of p-tert-butyl-tetrathiacalix[4]arene. X-ray diffraction studies revealed that in the solid state whereas p-tert-butyl-tetrathiacalix[4]arene forms inclusion complexes with solvent molecules, tetrathiacalix[4]arene undergoes self-inclusion leading to trimeric units. The same behaviour in the crystalline phase was also demonstrated for calix[4]arene.
 |  |  |
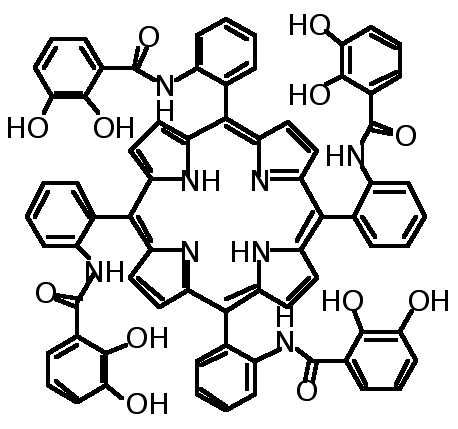
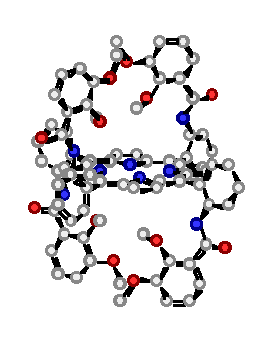
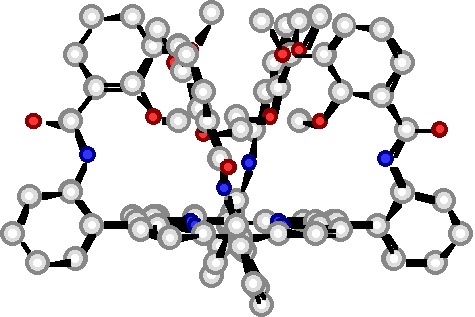
"Design, synthesis and structural studies on polynucleating ligands based on atropoisomerism of catechol bearing porphyrins"
C. DREXLER, M. W. HOSSEINI, J.-M. PLANEIX, G. STUPKA, A. DE CIAN, J. FISCHER, Chem. Comm. 1998, 689-690.(download .pdf)
Abstract : High yield syntheses of all four atropoisomers of the meso-tetrakis(o-catecholamidophenyl)porphyrin was achieved. All four atropoisomers were isolated and characterised by NMR spectroscopy. For the methyl protected 2abab and 2a4 atropoisomers obtained as water and chloroform solvates respectively, X-ray analysis was used to assign their structure.
 |  |  |


